Integrin Biology
The cytokine transforming growth factor β (TGFβ) is one of the key mediators in the immune system and has been studied extensively. Multiple clinical studies have, and continue to be, conducted on experimental therapies designed to inhibit its effect.
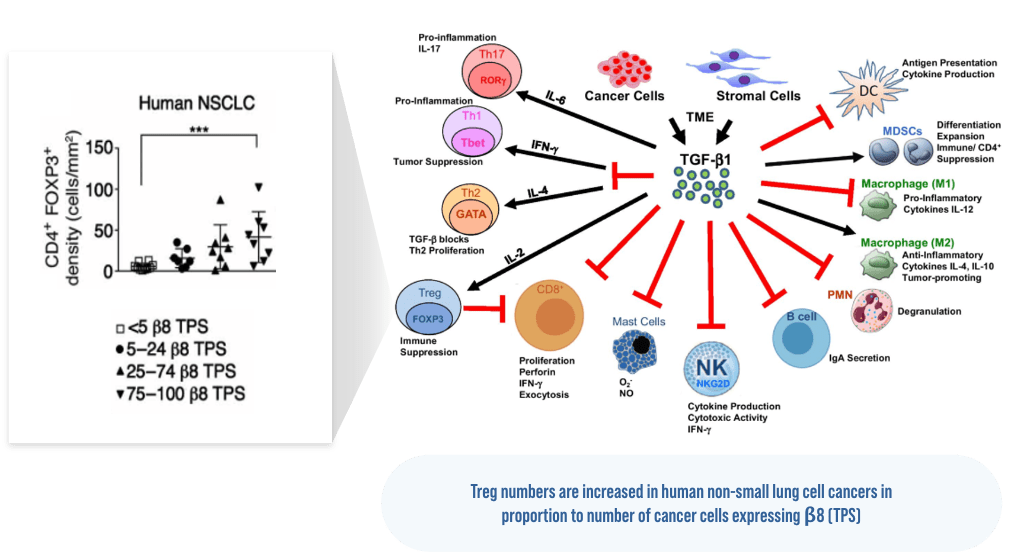
Seed, et al. A tumor-specific mechanism of T reg enrichment mediated by the integrin αvβ8, Science Immunology. 2021; 6:8.
Kim, et al. Novel therapies emerging in oncology to target the TGF-β pathway, Journal of Hematology & Oncology. 2021; 14:4.
TGFβ is a multifunctional cytokine involved in many cellular processes, including cell growth and differentiation, immune responses, wound healing, and tissue repair. TGFβ plays a key role in fibrosis and also promotes cancer growth and metastasis via its effects in the tumor microenvironment (TME). The integrins αvβ6 and αvβ8 are expressed by cancer cells, and αvβ6 is also expressed on epithelial cells in fibrotic diseases. These integrins enable TGFβ to exert its biologic effects by releasing it from its latent complex. The goal of blocking these integrins is to inhibit the deleterious effects of TGFβ. A number of other preclinical and early clinical stage programs are testing this approach of inhibiting αv integrins.
TGFβ is thought to promote growth and metastases of established tumors, inducing immunosuppression, fibrosis, blood vessel growth, and changes in tumor cells themselves. Some tumor cells of human epithelial malignancies express αvβ8, leading to activation of TGFβ in the tumor microenvironment. Overexpression of αvβ8 by tumor cells and expression of TGFβ in tumors has been linked to poor clinical outcomes.
Corbus is developing CRB-601 for treatment of solid tumors in combination with existing therapies.
CRB-601 is an anti-αvβ8 mAb and potent at picomolar concentrations in inhibiting activation of TGFβ. C6D4, the parent mAb of CRB-601, has single agent activity as well as synergistic activity when combined with an anti-PD1 mAb in syngeneic mouse tumor models. CRB-601 was licensed from University of California San Francisco (UCSF) and invented by Dr. Stephen Nishimura and his team.

CRB-601 will be studied for the treatment of solid tumors in combination with existing therapies, including checkpoint inhibitors (CPIs). CPIs are drugs that block proteins called checkpoints that are made by some immune system and cancer cells. Currently, only 44% of cancer patients are eligible for CPIs, and only 12.5% of all treated cancer patients respond. Among patients who respond to CPIs, disease progression often occurs due to resistance mechanisms. Significant unmet need remains for greater and more durable responses to CPIs.
We believe that the combination of CRB-601 and an anti-PD-1 mAb, a type of CPI, has the potential to provide greater and more durable anti-tumor responses than either agent alone. The solid tumors program continues to advance toward the clinic with first patient dosed anticipated in first half of 2023 following filing of an IND.
In inflammatory conditions, TGFβ worsens inflammation and prevents it from resolving. It is a hallmark of many chronic inflammatory and fibrotic diseases. In fibrotic conditions, TGFβ is commonly found at the site of inflammation where it acts as one of the most potent pro-inflammatory cytokines and promotes the accumulation of extracellular matrix that is a hallmark of fibrosis.
The αvβ6 and αvβ8 integrins have been implicated in fibrotic diseases and in cancers of epithelial cell origin. Targeting these integrins at the same time is a rational approach to treating fibrotic diseases, including idiopathic pulmonary fibrosis (IPF), and carcinomas.
Corbus expects that CRB-602 may provide potential therapeutic benefit in fibrotic diseases and cancer.
CRB-602 was developed to specifically inhibit both αvβ6 and αvβ8. Corbus continues to explore development pathways for CRB-602.
αvβ6 is more highly expressed in fibrotic areas of the lungs for people with idiopathic pulmonary fibrosis than in non-fibrotic areas of their lungs or normal lung.
Increased Expression of αvβ6 in Fibrotic
Areas in the Lung of IPF Subjects
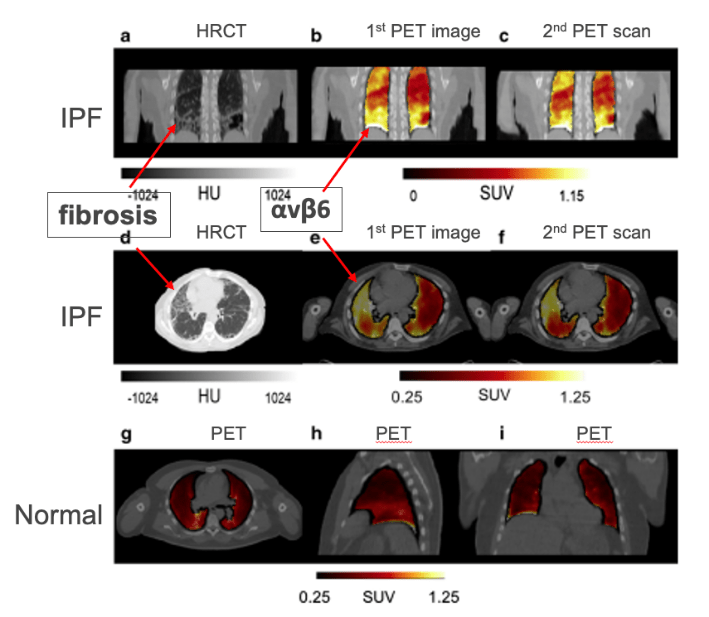
Lukey, et al. Clinical quantification of the integrin αvβ6 by [18 F]FB-A20FMDV2 positron emission tomography in healthy and fibrotic human lung (PETAL Study), European Journal of Nuclear Medicine and Molecular Imaging. 2020; 47:974.

